posted December 3, 2001 |
Written by Edward R. D. Scott |
The mixtures of oxygen isotopes in the Earth, Mars, and the asteroids differ slightly. If we knew why they differ we could learn more about the origin of asteroids and planets and the formation of the solar system. My colleague Sasha Krot and I describe one solution to part of this puzzle. We show how particles in primitive meteorites could have formed from gas and dust close to the Sun. This causes the particles to acquire different mixtures of oxygen isotopes from diverse stars that were ancestors to our own. Planets and asteroids inherited slightly different mixtures of oxygen atoms because they formed from materials like those in primitive meteorites.
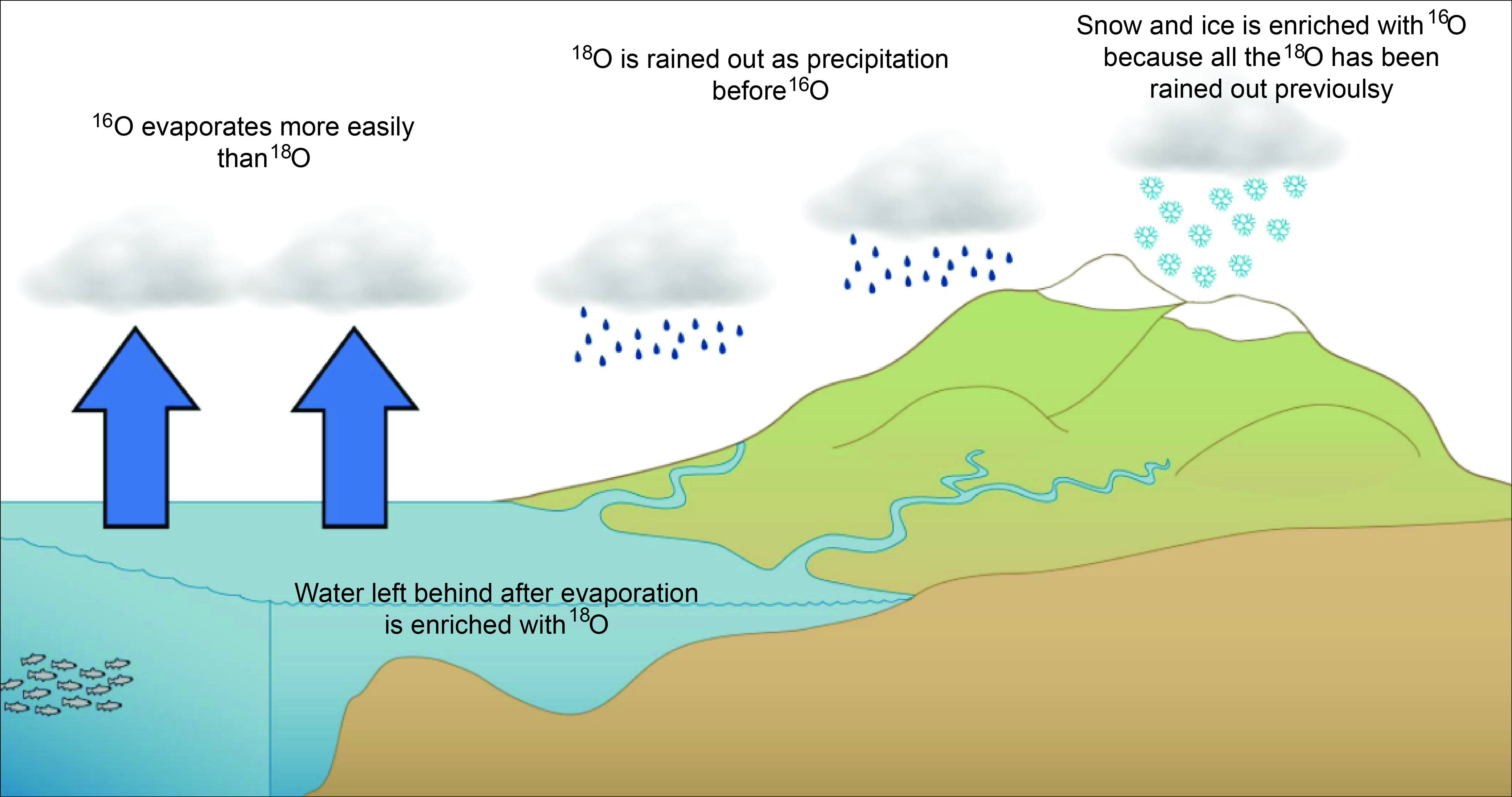
There are three known stable isotopes of oxygen (8 O): 16 O, 17 O, and 18 O. Radioactive isotopes ranging from 11 O to 26 O have also been characterized, all short-lived. Oxygen has seventeen known isotopes, all ranging widely from O-12 to O-28, however only three are stable and non-radioactive - O-16, O-17, and O-18. All other isotopes of oxygen do not last for long, due to having half-lives ranging from nanoseconds to seconds ('Isotopes of the Element Oxygen'). Apr 15, 2013 Stable isotopes Naturally occurring oxygen is composed of three stable isotopes, 16O, 17-O and 18-O, with 16 O being the most abundant (99.762% natural abundance). Known oxygen isotopes range in mass number from 12 to 24. The oxygen in the H 2 O is enriched in the lighter 16 O. This H 2 O condenses in clouds, falling on land as precipitation. Thus, H 2 O that is part of the terrestrial water cycle is enriched in the light 16 O isotope and sea water is enriched in the heavier 18 O isotope. Determining Past Climate Change - Oxygen Isotopes. Normal oxygen contains 8 protons, 8 neutrons (O 16); a small fraction (one in a thousand) of oxygen atoms contain 8 protons, 10 neutrons (O 18). This is an isotope of oxygen and is heavier than O 16; O 16 will evaporate more readily than O 18 since it is lighter.
The Informative Isotopes of Oxygen
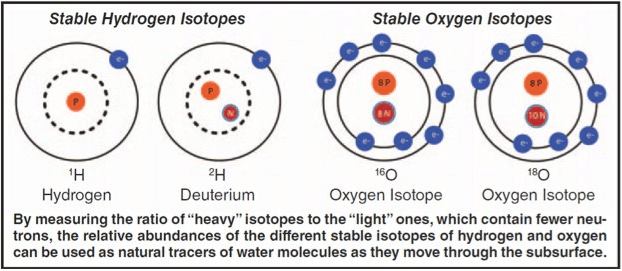
There are three stable varieties of oxygen atoms called isotopes that have the same chemical properties but masses that differ by ratios of 16:17:18. The mass of each atom depends on the total number of neutrons and protons that it contains. Most oxygen atoms contain 8 neutrons and 8 protons, which all have the same mass. In our solar system, about 1 in 500 oxygen atoms contains an extra neutron and is 17/16 times heavier. About 1 in 2000 contains two extra neutrons and is 18/16 times heavier. The three types of oxygen atoms are called 16O, 17O, and 18O.
The oxygen atoms in rocks that you pick up on Earth do not have identical proportions of 16O, 17O, and 18O atoms but the ratios of these isotopes follow a simple relationship that is controlled by their masses. Rocks with the same 18O / 16O ratio will have the same 17O / 16O ratios. If the 18O / 16O ratio in a given sample is, for example, 0.2% higher than it is in Standard Mean Ocean Water (SMOW), then the 17O / 16O ratio in that sample will depart from SMOW by half as much--in this example, it will be 0.1% higher.
The oxygen isotopic compositions of rocks from Earth, Mars, and the asteroid Vesta, the largest asteroid that melted, define three parallel lines on this plot of 17O / 16O vs. 18O / 16O. The lines are parallel because on each body the oxygen isotopes were separated according to their masses, when the rocks formed. Cosmochemists measure the 18O / 16O and 17O / 16O ratios in terms of deviations in parts per thousand from a standard (delta 18O and delta 17O). The usual standard is mean ocean water, abbreviated SMOW, for Standard Mean Ocean Water. Pure 16O would plot at -1000 parts per thousand on both axes. |
If you go to Mars and repeat the experiment you will find that the oxygen isotopic ratios follow the same relationship, but with one important difference. When you compare an Earth rock to a Mars rock with the same 18O / 16O ratio, you will find that the Martian rock has a slightly higher 17O / 16O ratio, as shown in the diagram above. The difference is small, about 3 parts in 10,000, but significant because oxygen is the only element that shows this effect, and because geological processes on the two planets cannot be responsible for the difference. (On Vesta, the largest melted asteroid, the corresponding 17O / 16O ratio is about 3 parts in 10,000 lower, and on the Moon, the isotopic ratios appear to be identical to terrestrial values: any difference is less than 0.2 parts per 10,000.)
The anomalous behavior of oxygen in extraterrestrial materials was not discovered originally in Martian rocks. It was discovered in chondritic meteorites. Chondrites come from asteroids that were never melted or heated significantly, but contain particles that formed at high temperatures (~1500 oC) when the solar system was growing from a disk of dust and gas called the solar nebula. These particles show enormous oxygen isotopic anomalies (also called 'mass-independent' isotopic effects). Two types of particles are present: those containing abundant oxides of calcium and aluminum, called calcium-aluminum-rich inclusions or CAIs, which show the largest oxygen isotopic effects, and the much more abundant variety composed largely of oxides of iron, magnesium and silicon called chondrules. Most of the chondrules and some of the CAIs formed from millimeter-sized globs of molten material that cooled in a few hours or less in the solar nebula before the planets formed.
Mineralogical map of a calcium-aluminum-rich inclusion (CAI) in the Efremovka carbonaceous chondrite [Data link]. In this image, obtained with an electron microprobe, the square and diamond-shaped, purplish grains are spinel, an aluminum-rich mineral. The light-green and green areas are crystals of minerals rich in both calcium and aluminum. Red area is the surrounding rock, which consists mostly of minerals rich in magnesium and iron. CAIs are thought to be among the very first solids to form in the solar nebula. |
| Microscopic view of chondrules in the meteorite Dhajala [Data link]. White grains are metallic iron-nickel and rounded gray objects are chondrules. The width of this photomicrograph is 2.7 millimeters. |
The oxygen isotopic compositions of CAIs and chondrules are different from those of Earth rocks in two important ways. If two chondrules or CAIs have 18O / 16O ratios that differ by 1%, their 17O / 16O ratios will also differ by 1% (not 0.5%), so their oxygen isotopic variations can be understood simply in terms of addition or loss of 16O atoms. CAIs and chondrules are also different because they show very large variations in their oxygen isotopic ratios of up to 5%. CAIs consistently have low 17O / 16O and 18O / 16O ratios whereas chondrules have diverse oxygen isotopic ratios that are much closer to those in Earth and Mars (see diagram below). Earth and Mars probably have different proportions of oxygen isotopes because they formed from different mixtures of chondrules and CAIs. But we don't know exactly how CAIs and chondrules formed and what caused their oxygen isotopic variations.
Plot showing the 18O / 16O and 17O / 16O ratios in chondrules and CAIs in meteorites. These particles define a line with much steeper slope than the Earth line consistent with loss or addition of 16O. Note that the variations in oxygen isotopic ratios are much larger than those shown by rocks from the Earth, Mars, and Vesta (see diagram above). |
Ideas for the Origin of Oxygen Isotopic Variations
The origin of the oxygen isotopic variations in solar system materials has been a major puzzle for planetary scientists since they were discovered nearly 30 years ago. To help solve it, the Genesis spacecraft was launched in August, 2001 to measure the proportions of oxygen isotopes in the stream of atoms ejected from the Sun into space. NASA hopes that a more accurate determination of the oxygen isotopic composition of the Sun, and hence the solar nebula, will provide a test for different theories for the origin of the oxygen isotopic variations among CAIs, chondrules, asteroids and planets.
Oxygen Isotopes And Temperature
One class of theories attributes the oxygen isotopic variations in CAIs and chondrules to differences in the oxygen isotopic compositions of the grains that existed in interstellar space prior to the formation of our solar system. The oxygen atoms in our solar system formed in a large number of stars that long before our solar system was born. The first stars in the Galaxy that were heavy enough to make oxygen atoms made almost pure 16O. Later generations of stars made progressively larger proportions of 17O and 18O from the oxygen in the ashes of the older stars. Don Clayton (Clemson University) argued that oxygen in interstellar oxides of elements such as silicon, magnesium, and iron is older and richer in 16O than the oxygen in interstellar ices. If so, solar nebula materials could have inherited 16O variations from these two types of pre-solar materials.
How could oxygen isotopic variations in pre-solar materials have been transferred into CAIs and chondrules? Robert Clayton at the University of Chicago, who with coworkers discovered the oxygen anomalies in meteorites, suggested that CAIs and chondrules formed from 16O-rich, pre-solar dust derived from interstellar grains containing silicon, magnesium, and oxygen that subsequently exchanged oxygen atoms with an 16O-poor gas made from interstellar ices. One strong point of this model is that it can explain why oxygen is the only element that shows widespread mass-independent isotopic variations. Oxygen is the only element that is abundant in both the gas and dust of the solar nebula over a wide temperature range (~ -100 to 1500 oC).
A second theory suggests that the 16O variations in CAIs and chondrules were not inherited from stars but were produced in the hot gaseous solar nebula by a chemical process capable of generating gaseous molecules with mass-independent isotopic variations. Such a process isn't known, but Mark Thiemens at the University of California, San Diego argues that since mass-independent oxygen isotopic effects are found in the Earth's atmosphere, they may also have been generated in the solar nebula gas. Thus, Thiemens attributes the 16O variations in CAIs and chondrules to their formation from solids that condensed from a gas in the solar nebula that had been enriched in 16O by a chemical process.
What fascinated me about the oxygen isotopic mystery was that neither Robert Clayton's model nor Thiemens' model appeared to be completely consistent with our ideas about the origins of CAIs. Clayton's model attributes the 16O-rich nature of CAIs solely to presolar solids, whereas Thiemens' model attributes them solely to solids that condensed from solar nebula gas. Yet the properties of CAIs suggest that both mechanisms were involved. Analyses of rare-earth elements in some CAIs suggest that they condensed from solar nebula gas, contrary to Clayton's oxygen model, which excludes any nebular condensates of oxides of calcium, aluminum, and silicon from meteorites. A few rare CAIs (a subset of so-called FUN CAIs) appear to have formed as simple evaporative residues of presolar solids, contrary to Thiemens' model.
New Ideas
Sasha Krot and I suggest that there is only one plausible reason why solar nebula condensates and evaporative residues of pre-solar dust could be similarly enriched in 16O. Condensation must have occurred in a region where the gaseous oxygen was dominated by oxygen from evaporated 16O-rich pre-solar dust. This could have happened if the dust was concentrated relative to nebular gas before the dust was evaporated. Dust concentration prior to evaporation is commonly invoked to explain certain chemical features of suspected nebular condensates but seldom to explain isotopic effects. With a few plausible assumptions about the composition of the interstellar components, a simple model shows that CAIs could have condensed from a gas formed by enriching dust relative to solar nebula gas by factors of ~10-50 or more. Chondrules require lower enrichment factors of 1-5.
We know that dust was concentrated relative to gas in the solar nebula and that rocky material was vaporized, but we don't know how and where. Pat Cassen at NASA Ames Research Center has proposed that dust was enriched relative to gas and vaporized as it fell into a hot region of the solar nebula. He calculates that a region extending well beyond the current orbit of Mars was so hot that almost all rocky material in it was vaporized. One problem with this model is that it is difficult to see how CAIs could cool rapidly in hours after they formed at high temperatures.
A second model for explaining how solids were vaporized to form CAIs and chondrules has been developed by Frank Shu and his colleagues at the University of California at Berkeley [See PSRD article: Relicts from birth of the Solar System.] In their model, which was initially developed to explain the formation of bipolar jets around accreting stars, the solar nebula is not hot enough to evaporate rocky material except at the innermost edges, close to the young Sun (well inside Mercury's current orbit). In this region, the nebular gases are dragged into the young Sun by its magnetic field leaving solid residues accumulating at the midplane. According to Shu and colleagues, thermally processed grains--including chondrules and CAIs--were periodically hurled back to the outer regions of the solar nebula by a so-called x-wind.
Chondrules and CAIs may have been formed at the innermost edge of the disk close to the young Sun and been hurled outwards by the x-wind to cooler regions where they accreted. The oxygen isotopic anomalies in extraterrestrial materials probably result from thermal processing of 16O-rich interstellar dust and 16O-poor nebular gas close to the Sun. |
We suggest that the innermost edge of the solar nebula is a more plausible location for generating the 16O variations in chondrules and CAIs than the one proposed by Cassen because it provides an environment where CAIs (and chondrules) might cool rapidly--in hours. An 16O-rich gas would have formed if 16O-rich solids spiralled inwards and accumulated at the midplane close to the young Sun after the nebular gases were dragged into the Sun. Evaporation of these solids during a sudden burst of energy from the Sun could have generated 16O-rich gas. The idea that chondrules and CAIs formed very close to the Sun and not in the asteroid belt is a new concept, which has not been embraced by many meteorite researchers. However, certain chondrules in metal-rich chondrites discovered by Sasha Krot and coworkers have properties consistent with their formation close to the sun. [See PSRD article: Relicts from birth of the Solar System.]
If these ideas about the origin of the oxygen isotopic anomalies are supported by additional modeling and astronomical observations of young stars, we may have a plausible framework for understanding how chondrules and CAIs were formed and accumulated into asteroids, and planets. However, there will still be much to do to understand the life story of the oxygen atoms from their birth in stars and their transport in interstellar grains, chondrules, and CAIs into planets. Measurements of the oxygen isotopic composition of the Sun are unlikely to settle the oxygen isotope mystery because predictions of the two models overlap considerably. But measurements on the dirt and ices in comets and the moons of outer planets should help to solve it.
Oxygen Isotope With 9 Neutrons
| [ About PSRD | Archive | Search | Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |
psrd@higp.hawaii.edu
main URL is http://www.psrd.hawaii.edu/
Oxygen Isotopes in Meteorites
Abstract
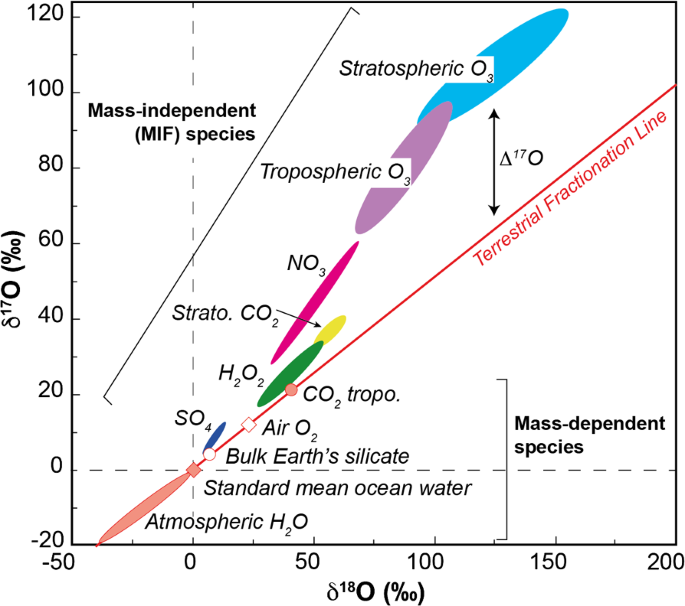
Oxygen isotope abundance variations in meteorites are very useful in elucidating chemical and physical processes that occurred during the formation of the solar system (Clayton, 1993). On Earth, the mean abundances of the three stable isotopes are 16O: 99.76%, 17O: 0.039%, and 18O: 0.202%. It is conventional to express variations in abundances of the isotopes in terms of isotopic ratios, relative to an arbitrary standard, called SMOW (for standard mean ocean water), as follows:The isotopic composition of any sample can then be represented by one point on a 'three-isotope plot,' a graph of δ17O versus δ18O. It will be seen that such plots are invaluable in interpreting meteoritic data. Figure 1 shows schematically the effect of various processes on an initial composition at the center of the diagram. Almost all terrestrial materials lie along a 'fractionation' trend; most meteoritic materials lie near a line of '16O addition' (or subtraction). (4K)Figure 1. Schematic representation of various isotopic processes shown on an oxygen three-isotope plot. Almost all terrestrial materials plot along a line of 'fractionation'; most primitive meteoritic materials plot near a line of '16O addition.' The three isotopes of oxygen are produced by nucleosynthesis in stars, but by different nuclear processes in different stellar environments. The principal isotope, 16O, is a primary isotope (capable of being produced from hydrogen and helium alone), formed in massive stars (>10 solar masses), and ejected by supernova explosions. The two rare isotopes are secondary nuclei (produced in stars from nuclei formed in an earlier generation of stars), with 17O coming primarily from low- and intermediate-mass stars (<8 solar masses), and 18O coming primarily from high-mass stars (Prantzos et al., 1996). These differences in type of stellar source result in large observable variations in stellar isotopic abundances as functions of age, size, metallicity, and galactic location ( Prantzos et al., 1996). In their paper reporting the discovery of 18O in the Earth's atmosphere, Giauque and Johnston (1929) refer to nonuniform distribution of oxygen isotopes as a 'remote possibility,' whereas Manian et al. (1934) sought to find variations in oxygen isotope abundances in meteorites as evidence for an origin outside the solar system.In addition to the abundance variations due to nuclear processes, there are important isotopic variations produced within molecular clouds, the precursors to later star-formation. The most important process is isotopic self-shielding in the UV photodissociation of CO (van Dishoeck and Black, 1988). This process results from the large differences in abundance between C16O, on the one hand, and C17O and C18O on the other. Photolysis of CO occurs by absorption of stellar UV radiation in the wavelength range 90-100 nm. The reaction proceeds by a predissociation mechanism, in which the excited electronic state lives long enough to have well-defined vibrational and rotational energy levels. As a consequence, the three isotopic species - C16O, C17O, and C18O - absorb at different wavelengths, corresponding to the isotope shift in vibrational frequencies. Because of their different number densities, the abundant C16O becomes optically thick in the outermost part of the cloud (nearest to the external source of UV radiation), while the rare C17O and C18O remain optically thin, and hence dissociate at a greater rate in the cloud interior. The differences in chemical reactivity between C16O molecules and 17O and 18O atoms may lead to isotopically selective reaction products. This scenario has been suggested to explain meteoritic isotope patterns, as discussed below (Yurimoto and Kuramoto, 2002).Stable isotope abundances in meteoritic material provide an opportunity to evaluate the thoroughness of mixing of isotopes of diverse stellar sources. Molybdenum presents a good test case: it has seven stable isotopes, derived from at least three types of stellar sources, corresponding to the r-process, s-process, and p-process. Presolar silicon carbide grains, extracted from primitive meteorites, contain molybdenum that has been subject to s-process neutron capture in red-giant stars, resulting in large enrichments of isotopes at masses 95, 96, 97, 98, and severe depletions (up to 100%) of isotopes at masses 92 and 94 (p-process) and 100 (r-process) (Nicolussi et al., 1998). Complementary patterns have been found in whole-rock samples of several meteorites, with >1,000-fold smaller amplitude, suggesting the preservation of a small fraction of the initial isotopic heterogeneity ( Yin et al., 2002; Dauphas et al., 2002). Oxygen is another element for which primordial isotopic heterogeneity might be preserved. This is discussed further below.It would be highly desirable to have samples of oxygen-rich mineral grains that have formed in stellar atmospheres and have recorded the nucleosynthetic processes in individual stars. Similar samples are already available for carbon-rich grains, in the form of SiC and graphite, primarily from asymptotic giant branch (AGB) stars and supernovae (Anders and Zinner, 1993). These presolar grains have provided a wealth of detailed information concerning nucleosynthesis of carbon, nitrogen, silicon, calcium, titanium, and heavier elements (see Chapter 1.02). It is thought that such carbon-rich minerals should form only in environments with C/O>1, as in the late stages of AGB evolution, or in carbon-rich layers of supernovae. By analogy, one would expect to form oxide and silicate minerals in environments with C/O<1, as is common for most stars. Indeed there is evidence in infrared spectra for the formation of Al2O3 (corundum) and silicates, such as olivine (Speck et al., 2000) around evolved oxygen-rich stars. However, searches for such grains in meteorites have yielded only a very small population of corundum grains, a few grains of spinel and hibonite, and no silicates ( Nittler et al., 1997). The observed oxygen isotopic compositions of presolar corundum grains show clear evidence of nuclear processes in red-giant stars, and have had significant impact on the theory of these stars ( Boothroyd and Sackmann, 1999).There are several possible reasons for the failure to recognize and analyze large populations of oxygen-rich presolar grains:(i) they may not exist: oxygen ejected in supernova explosions may not condense into mineral grains on the short timescale available;(ii) they may be smaller in size than can be detected by applicable techniques (∼0.1 μm); and(iii) they may be destroyed in the laboratory procedures used to isolate other types of presolar grains.

- Publication:
- Pub Date:
- December 2003
- DOI:
- 10.1016/B0-08-043751-6/01063-X
- Bibcode:
- 2003TrGeo...1..129C
